Ivonescimab - Promising Efficacy in NSCLC
- Feb 3, 2025
- 3 min read
Ivonescimab - Promising Efficacy in NSCLC
Ivonescimab, also known as AK112, is a novel bispecific monoclonal antibody that simultaneously targets programmed cell death protein 1 (PD-1) and vascular endothelial growth factor (VEGF). It has been developed to harness the synergistic effects of immune checkpoint blockade and antiangiogenesis for the treatment of cancer. Developed by Akeso Biopharma, this dual-targeting mechanism positions ivonescimab as a promising therapeutic option for solid tumors, particularly non-small cell lung cancer (NSCLC) and other VEGF-driven malignancies. In May 2024, ivonescimab in combination with chemotherapy was approved in China for treating EGFR-mutated non-squamous NSCLC patients who have progressed after tyrosine kinase inhibitor (TKI) therapy.
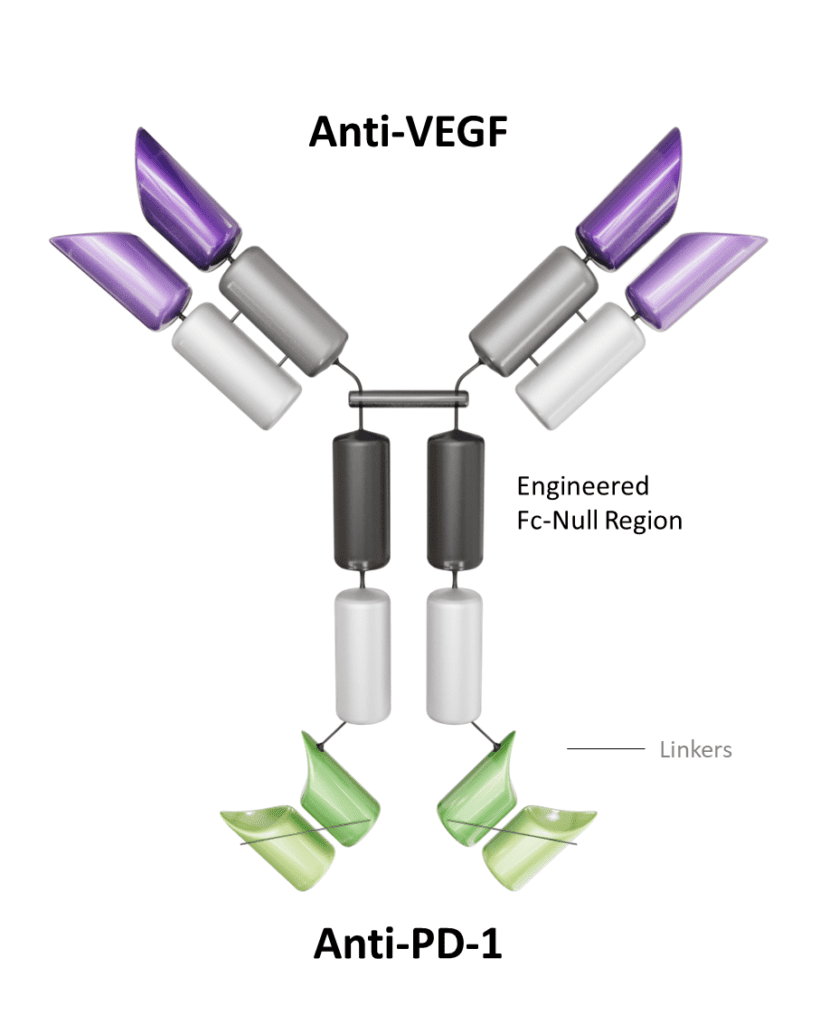
Mechanism of Action
Ivonescimab combines two key therapeutic strategies in oncology:
PD-1 Blockade: By targeting PD-1, ivonescimab reactivates T cells that have been rendered dysfunctional by tumor-induced immune suppression. This enables the immune system to mount an effective antitumor response.
VEGF Inhibition: Simultaneously, ivonescimab inhibits VEGF, a protein that promotes tumor angiogenesis. This disruption of blood vessel formation limits the tumor’s access to oxygen and nutrients, thereby impeding its growth and metastasis.
The dual action of ivonescimab addresses both immune evasion and angiogenesis, potentially offering improved efficacy over single-agent PD-1 inhibitors.
Pharmacokinetics and Pharmacodynamics
According to available data, ivonescimab demonstrates a prolonged half-life and linear pharmacokinetics across a range of doses. It achieves adequate systemic exposure with manageable toxicity profiles. Its pharmacodynamic effects are mediated by the simultaneous inhibition of the PD-1/PD-L1 pathway and VEGF signaling, which reduces immunosuppression and angiogenesis, respectively.
Usual Dosing
In clinical trials, ivonescimab has been administered intravenously at doses ranging between 10 mg/kg and 30 mg/kg every 2–3 weeks. The dosing schedule may vary depending on the specific indication, tumor type, and patient response. Further studies are ongoing to determine the optimal therapeutic dose.
Kidney and Hepatic Impairment:
As of the current data, specific dosing adjustments for ivonescimab in patients with renal or hepatic impairment have not been clearly established. Further pharmacokinetic studies in these populations are needed to provide more comprehensive recommendations.
Administration:
Ivonescimab is administered as an intravenous infusion. Careful monitoring is recommended during administration, especially for infusion-related reactions and signs of immune-related adverse events.
Clinical Efficacy:
Ivonescimab has shown promising efficacy particularly in EGFR-mutated NSCLC patients who have progressed after tyrosine kinase inhibitor (TKI) therapy. In a phase III trial, ivonescimab plus chemotherapy significantly improved progression-free survival with tolerable safety profile in patients with NSCLC who previously underwent EGFR-TKI treatment and may offer a new treatment option for patients with TKI resistance. JAMA

Safety Profile
Ivonescimab has been generally well-tolerated in clinical trials. Common adverse events include fatigue, hypertension, and proteinuria, which are expected from VEGF-targeting agents. Immune-related adverse events, such as pneumonitis and colitis, may also occur due to PD-1 inhibition. However, the safety profile is consistent with those of other agents in its class, and no unexpected toxicities have been reported to date.
Comparison with Pembrolizumab
Mechanism of Action: Ivonescimab combines PD-1 blockade with VEGF inhibition, addressing both immune evasion and angiogenesis. Pembrolizumab targets PD-1 alone and does not inhibit VEGF, lacking an antiangiogenic effect.
Clinical Efficacy: Ivonescimab has shown promising efficacy in patients with NSCLC who progressed on TKIs. The dual mechanism of ivonescimab may improve efficacy, particularly in VEGF-driven cancers.
Toxicity Profiles: Pembrolizumab is associated primarily with immune-related toxicities such as pneumonitis and colitis. Ivonescimab introduces VEGF-related toxicities, such as hypertension and proteinuria, alongside immune-related adverse events.
Potential Advantages: Ivonescimab's dual targeting could provide an edge in cancers resistant to prior PD-L1 therapy. VEGF inhibition may enhance outcomes in aggressive or angiogenesis-driven cancers where pembrolizumab alone might not be as effective.
NCCN Guidelines on Ivonescimab for Non-Small Cell Lung Cancer
As of now, ivonescimab is not explicitly included in the NCCN guidelines for NSCLC. However, its dual mechanism of action and emerging clinical data suggest it may become a valuable option for advanced NSCLC and other VEGF-driven cancers in future guideline updates, pending additional evidence from ongoing phase II/III trials. Ivonescimab with chemotherapy is approved in China for treating EGFR-mutated non-squamous NSCLC patients who have progressed after tyrosine kinase inhibitor (TKI) therapy.
Conclusion
Ivonescimab represents an innovative approach to cancer therapy by integrating immune checkpoint blockade with antiangiogenesis. Its dual-targeting strategy offers a potentially superior therapeutic benefit for patients with solid tumors, particularly in heavily pretreated NSCLC populations. While further studies are required to validate its efficacy and safety, ivonescimab holds promise as a next-generation therapeutic option that could reshape the treatment landscape for advanced cancers.



Comments